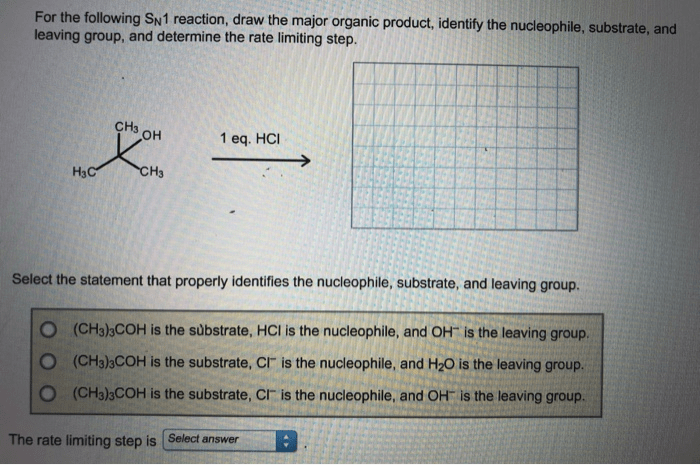
For the sn1 reaction draw the major organic product – In the realm of organic chemistry, the SN1 reaction stands as a captivating subject, inviting us to delve into its intricacies and uncover the secrets behind the formation of its major organic product. This analysis embarks on a journey to unravel the mechanisms, factors, and applications that govern this intriguing reaction, providing a comprehensive understanding of its fundamental principles and practical significance.
The SN1 reaction, characterized by its unimolecular nucleophilic substitution pathway, unfolds in a stepwise fashion, initiating with the ionization of the substrate to form a carbocation intermediate. This highly reactive species subsequently undergoes nucleophilic attack, leading to the formation of the final product.
Understanding the factors that influence the product distribution, such as solvent polarity, nucleophile strength, and substrate structure, empowers chemists to manipulate reaction conditions and favor the desired outcome.
SN1 Reaction
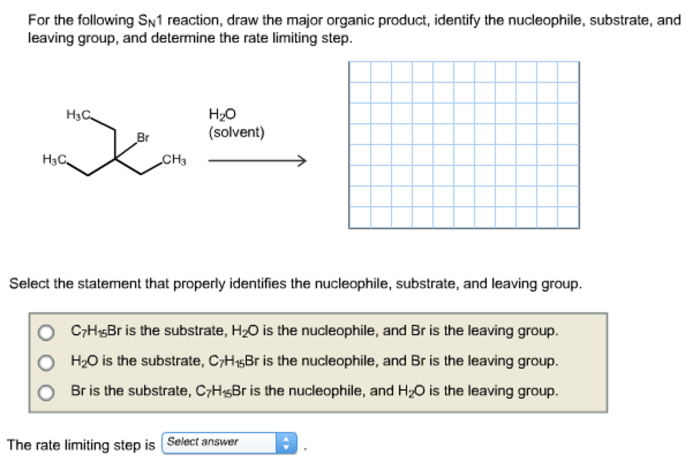
The SN1 reaction is a unimolecular nucleophilic substitution reaction in which the rate-determining step is the ionization of the substrate to form a carbocation intermediate. This intermediate is then attacked by a nucleophile to form the product. The SN1 reaction is typically favored by polar aprotic solvents and by substrates that are able to form stable carbocations.
Mechanism of the SN1 Reaction
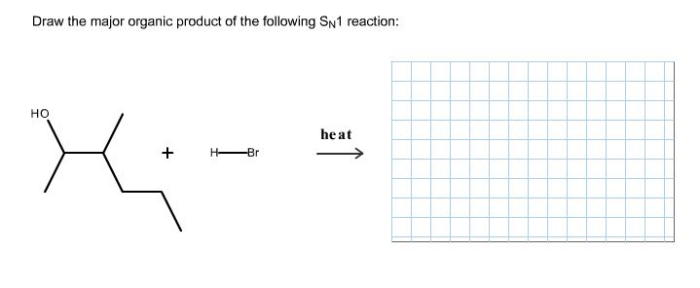
The SN1 reaction proceeds through a two-step mechanism. In the first step, the substrate ionizes to form a carbocation intermediate. This step is slow and rate-determining. In the second step, the carbocation intermediate is attacked by a nucleophile to form the product.
This step is fast and irreversible.
Factors Affecting the Product Distribution, For the sn1 reaction draw the major organic product
The product distribution of the SN1 reaction is affected by a number of factors, including the nature of the substrate, the nucleophile, and the solvent.
- The nature of the substrate:Substrates that are able to form stable carbocations are more likely to undergo SN1 reactions. This is because the formation of the carbocation intermediate is the rate-determining step in the SN1 reaction.
- The nature of the nucleophile:Nucleophiles that are strong and unhindered are more likely to attack carbocations. This is because strong nucleophiles are more likely to react with the carbocation intermediate, and unhindered nucleophiles are more likely to be able to reach the carbocation intermediate.
- The nature of the solvent:Polar aprotic solvents are more likely to favor SN1 reactions. This is because polar aprotic solvents are able to solvate the ions that are formed in the SN1 reaction, which makes the reaction more favorable.
Regioselectivity and Stereoselectivity
The SN1 reaction can be either regiospecific or stereospecific. Regiospecific reactions are those in which the nucleophile attacks the carbocation intermediate at a specific carbon atom. Stereoselective reactions are those in which the nucleophile attacks the carbocation intermediate in a specific stereochemical orientation.
Applications of the SN1 Reaction
The SN1 reaction is a useful tool for the synthesis of a variety of organic compounds. Some of the most common applications of the SN1 reaction include:
- The synthesis of alkyl halides:The SN1 reaction can be used to synthesize alkyl halides by reacting an alcohol with a strong acid.
- The synthesis of alkenes:The SN1 reaction can be used to synthesize alkenes by reacting an alkyl halide with a strong base.
- The synthesis of ethers:The SN1 reaction can be used to synthesize ethers by reacting an alcohol with an alkyl halide.
Q&A: For The Sn1 Reaction Draw The Major Organic Product
What is the key step in the SN1 reaction mechanism?
The ionization of the substrate to form a carbocation intermediate.
How does solvent polarity affect the SN1 reaction?
Polar solvents stabilize the carbocation intermediate, promoting the SN1 pathway.
Can SN1 reactions exhibit stereoselectivity?
Yes, under certain conditions, SN1 reactions can exhibit stereoselectivity, leading to the formation of specific stereoisomers.